









© 2023 Ice & Snow Technologies, Inc. Phone: (509) 525-3197





Leading North American Snow Industry Consultant
Deicer Product Analysis, Testing, and Development Expert
Salt Brine Production Systems Design, Build and Training
What is a Deicer?
by Dale Keep, Road and Highway Ice Control and Snow Removal Industry Expert
Deicers are generally known to be products that are highly soluble in water at low temperatures and exhibit a
eutectic temperature (eutectic temperature defines the lowest temperature at which a solution can melt ice or
snow) preferably several degrees lower than the ambient temperature encountered in the ice or snow event. They
are rated by their potential for chemical ice melting capacities and can be further defined by their low
temperature solubilities in water, freezing point depression versus concentration, and its freeze point at different
solutions. The less chemical in the solution, the higher the freezing point.
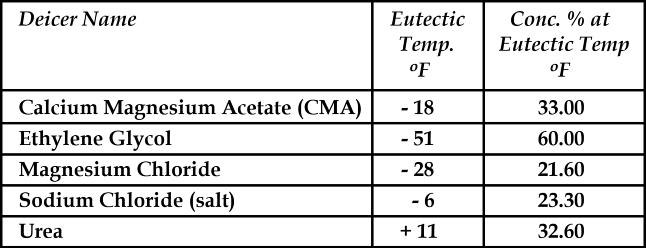
The table below shows the eutectic temperature of deicing chemicals at their optimum solution percentage.
How A Deicer Works
All deicers are either endothermic (absorbs heat) or exothermic (gives off heat). Sodium chloride is endothermic
(absorbs heat); therefore, it absorbs heat from the ice water it makes while melting snow or ice. This lowers the
temperature of the water and its freezing point to below freezing. This explains why salt is used on the ice in an
ice cream maker to freeze the ice cream in the maker while the saltwater solution does not freeze. The salt added
to the ice melts the ice, lowers the freezing point of the water and results in a solution cold enough to freeze the
ice cream. Also, if the ice cream is not freezing, simply add more salt, absorb more heat, and lower the
temperature of the solution until it does freeze the ice cream. As a result of this endothermic action, salt becomes
less effective at lower temperatures as the water becomes too cool to remain in a liquid state. Exothermic
materials (Calcium Chloride) give off heat and raise the temperature of the water to a temperature sometimes way
above the freezing point of water. This heating action will melt very well if enough chemical is applied, but if
the air temperature for example is 25°F one does not have to think too long about what happens to the heat of the
brine after the chemicals exothermic heating action stops. In addition to being either endothermic or exothermic,
deicers work in one of two ways.
The first is by dissolving with the ice or snow and simply melting the mass of material from the top down and
creating slush containing a deicer. This slush can be plowed and will not freeze on the pavement with existing
conditions. If it becomes too dry (less than 15% water) it tends to pack and bond to the pavement. You can
estimate when you are at or near the 15% water content of the slush by watching how it comes off vehicle tires.
Vehicles will throw slush sideways and eventually off the road when the water content is high. When the water
content approaches 15% moisture, tires will pick it up and throw it backwards.
The second is by penetrating the ice or snow with a solid deicer and then dissolving that deicer at the surface
spreading a thin layer of brine between the snow or ice and the pavement. This penetration process breaks the
ice and pavement bond and is more efficient in terms of quantities of deicers required and vehicle safety. Less
deicer is used with the penetration process because only a small mass of material needs to be melted, first in a
vertical channel and then at the ice-pavement interface while the deicer spreads. Liquid deicers if applied
correctly can work well for this.
The effectiveness of a deicer and by which of the two methods it is working can easily be assessed by observing
changes in the material on the road surface over time. If the penetration and bond breaking process is dominant,
the snow or ice will be cast from the road by vehicle tires or plowing to expose bare pavement which is wet with
deicer solution. When a deicer is melting at the surface (assuming sufficient deicer is applied) the snow or ice
will get wet on the surface, gradually turn to slush and then to solution. Providing it does not re-freeze the
solution then gradually drains or evaporates from the pavement eventually leaving a bare and dry surface. The
rate at which pavement dries depends on the relative humidity, precipitation, wind, sunlight, chemistry of the
deicer used, and traffic volume.